2100 Expert Security Pack
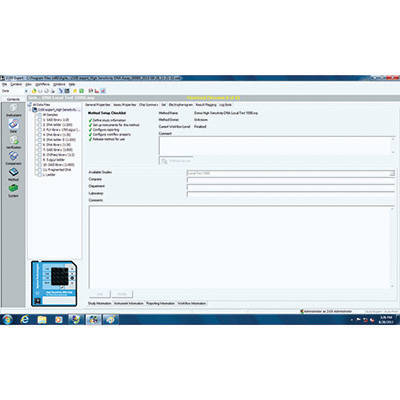
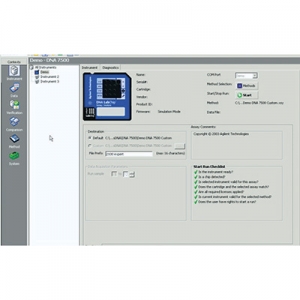
Elevate data analysis from GMP/GLP to 21 CFR Part 11 compliance with the 2100 Expert Security Pack as an add-on to the Bioanalyzer Expert Software. Additional user management, pre-validated methods, and access control help to comply with data security, integrity and traceability requirements mandated by FDA 21 CFR Part 11.
Easily create audit trails to track and prove user actions, and manage workflows for enhanced productivity.
Note: It is mandatory to purchase the 2100 Expert SW Laptop Bundle for operation.